Table Of Content

And when you read the FDA guidance, just about every reference involving User Needs is tied directly to Design Validation. Validation proves your medical device meets User Needs and intended uses. Keeping the DHF in a place where your entire product development project team can access it is also important. Throughout product development, your team will be contributing valuable content to include in the DHF, and accessing this same content to conduct product development.
Adopt a Unified Approach
Manor Lords is one of the more infrastructure-intensive city builders out there. For example, let’s say you want to start producing yarn — not even clothes, just yarn. You’ll need a livestock trader, a sheep farm, and a weaver’s workshop at a minimum. But each part of that process requires a family to be assigned to the building(s), and families require burgage plots to live on.
Why capturing User Needs sets the stage
Like the dev said on Steam, Manor Lords is “a citybuilder with battles,” not a grand army simulator. The advantage is having full access to the firmware and configuration files to be able to make changes. This guide explains the whole process to root Creality K1 Series and Ender-3 V3 Series and add features to your printer using Creality Helper Script. At $495, the DaVinci Resolve Micro Color Panel is significantly more affordable than its larger counterparts. This opens the door for a wider range of editors and colorists to experience the power of dedicated control panels and take their creative output to the next level.
What exactly are design controls?
Combination products: What role does packaging play? - Packaging Digest
Combination products: What role does packaging play?.
Posted: Thu, 30 Nov 2023 10:11:35 GMT [source]
Back in 1998, I started my career as a medical device product development engineer. The Office of Product Evaluation and Quality (OPEQ) reviews the Quality System design and manufacturing information in the PMA submission. OPEQ determines whether the manufacturer has described the processes in sufficient detail and make a preliminary determination of whether the manufacturer meets the QS requirements. If the manufacturer has provided an adequate description of the design and manufacturing process,a preapproval inspection can be initiated. Peter Knauer is a partner consultant with MasterControl's Quality and Compliance Advisory Services. He was most recently head of CMC operations for British Technology Group in the United Kingdom and he has held leadership positions for Protherics UK Limited and MacroMed.
Design controls
During later phases, a change will delay development and require more person power to achieve. To stay on track, ensure design inputs are clear, concise, and complete. In order to validate the design of your medical device, you need to build products. These products are to be built using production equivalent documentation and processes. Design Validation most definitely involves evaluation of products. And because of this, the process of transferring a medical device from product development to production (often referred to as “Design Transfer”) begins during Design Validation.

Design Controls are the process / system that ensures that a medical device is developed according to user needs, stakeholder requirements and demonstrates that it is safe and effective. Design Reviews are checkpoints throughout the design process that can prevent wasted time and money on a device that won’t work or meet requirements. Completing thorough, cross-functional reviews at each phase and taking necessary corrective actions can prevent issues down the road when it comes time for Design Verification and Design Validation (VnV). Risk Management is most commonly applied per the ISO standard, recognized by both FDA and EU.
The stuff I covered so far about understanding medical device product classification and quality systems is very important for you to have some grasp on as you pursue your new medical device idea. In fact, it is quite common for me to hear negative comments about design controls when I speak to product developers. I assure you that if you have bad feelings about design controls, it is likely because of the processes you are working within; design controls are what we do as prudent product developers. For each record, the language must reference the acceptance criteria that is laid out in the corresponding design input.
For example, a change in the intended use of the device will require validation. However, if a firm was making a design change in the material used in the device, then verification through analysis may only be required. The burden is on the firm to justify and document why verification only is appropriate in lieu of validation. In making the determination of the firm's ability to design, manufacture or process the device, OPEQ may issue an inspection assignment to the appropriate FDA district office.
Defining how to make your medical device through Design Outputs
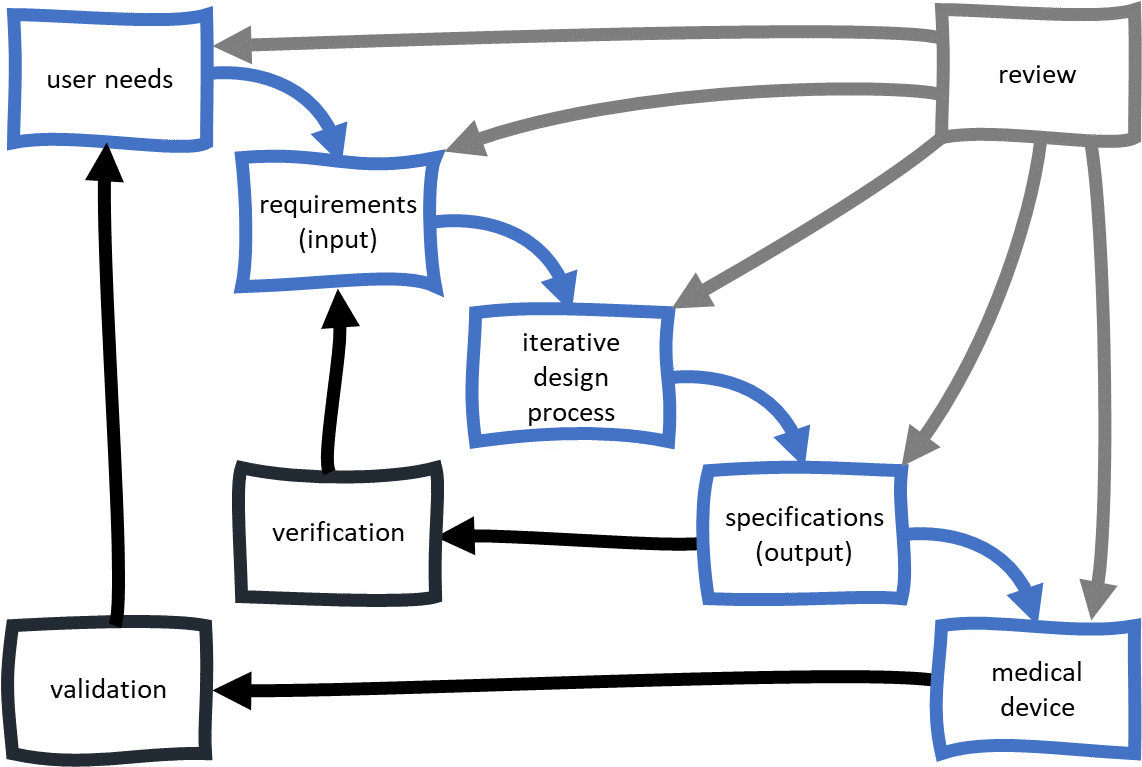
FDA does not mandate a waterfall approach for medical device product development and capturing Design Controls. In fact the FDA Design Control Guidance published in 1997 is very clear about the iterative nature during device development. It is an expected best practice that you integrate Design Controls and Risk Management during your medical device product development efforts. For Risk Management, be sure your approach is up-to-date and aligns with ISO 14971. Both FDA Design Controls regulations and ISO Design & Development requirements expect you to keep documentation and records throughout the product development process. I just need to spend a few minutes explaining what a quality system is and how this relates to your medical device product development efforts.
He specializes in technical guidance and product development efficiency for global organizations. An expert in software development, he is dedicated to helping customers drive quality product strategy. Design transfer is how you translate the device design into production, distribution, and installation specifications. After putting together designs and discovering any problems that will or may occur, you need to confirm the design is right and buildable.
While change is a healthy and necessary part of product development, quality can be maintained only if those changes are controlled and documented. As such, a key component of design control involves establishing procedures to identify, document, review, verify, validate, and approve design changes before they are implemented. You are not expected to maintain records of all changes proposed during the very early stages of the design process; however, all design changes made after the first design review must be documented. These records create a history of the evolution of the design, which can be useful for failure investigation and for facilitating the design of similar products in the future. Such records can also prevent repeat errors and the development of unsafe or ineffective designs. The number and types of reviews are project-dependent, so define them in the plan.
However, most post-production change control procedures may be too restrictive and stifle the development process. Firms may use a separate and less stringent change control procedure for pre-production design changes. While change is a healthy and necessary part of product development, quality can be ensured only if change is controlled and documented in the development process, as well as in the production process. Design validation involves the performance of clinical evaluations and includes testing under actual or simulated use conditions. Clinical evaluations can include clinical investigations or clinical trials, but they may only involve other activities. These may include evaluations in clinical or non-clinical settings, provision of historical evidence that similar designs are clinically safe, or a review of scientific literature.
On the one hand, an enhanced DE combining adaptive mutation operator and random crossover operator is proposed to improve the global search ability. On the other hand, the optimization method of initial population is proposed to solve the convergence efficiency problem of high-dimensional structure. The accuracy and efficiency of the proposed method are verified through the presentation of five representative cable-strut structures employing distinct design variables and equivalent force models. The computational efficiency and convergence accuracy of the method are also evaluated. This method is suitable for force finding of various cable-strut structures and can realize parametric design. It can dynamically show the accuracy change of iterative process and provide convenience for the initial prestress design of engineering designers.
A traceability matrix, which connects design inputs (requirements) to their related issues and tests, will be critical in verifying your device. While you can create a matrix using spreadsheets or similar, many device developers use specialized ALM tools to generate them automatically. These definitions need to be approved by the people outlined in your design planning document. Any changes made as a result of verification or validation (testing activities, covered later) will also need to be documented. What I mean by this is that very few use companies seem to adopt the terminology as described by FDA and ISO.


No comments:
Post a Comment